

How is acid / base (alkalinity) created in the human body?? and Does It Effect the pH of the Blood and Interstitium?
The following scientific discourse are twenty-five important points to understand the creation of hydrochloric acid (HCL) and sodium bicarbonate in the stomach lining, the ingestion of protein and sugar, and how acid/alkaline biochemistry, physiology and anatomy effects the blood pH and the interstitial fluid pH and their relationship to health, sickness and disease.
Unfortunately, conventional medical doctors and scientists do not understand how acid/base is created in the human body, and the onset of latent tissue acidosis of the interstitial fluids of the Interstitium.
Welcome to the 21st century and Dr. Young’s “New Biology.”
How is acid/base created by the stomach, and then the blood and interstitial fluids?
1) The parietal or cover cells of the stomach split the sodium chloride or salt of the blood. The sodium is used to bind with water and carbon dioxide to form the alkaline salt known as sodium bicarbonate or NaHCO3.
The biochemistry is: H20 + CO2 + NaCl = NaHCO3 + HCL.
2) For each molecule of sodium bicarbonate or NaHCO3 a molecule of hydrochloric acid or HCL is formed as an acidic waste product and secreted into the digestive system or stomach (held in the gastric pits of the stomach) to be eliminated. HCL is not produced for digesting food but is a waste product of sodium bicarbonate production for alkalizing the food and water you ingest.
3) The chloride ion from the sodium chloride (salt) binds to an acid or proton forming HCL as a waste product of sodium bicarbonate production that takes place throughout the day to alkalize your food and water ingested and the acids produced from metabolism.
4) When large amounts of acids including HCL enter the stomach from a rich animal protein meal, acid is withdrawn from the acid-base household.
The human body would die if the resulting alkalosis or NaHCO3 (the base flood) or base surplus created by the stomach was not taken up by the alkalizing glands that need these quick bases in order to build up their strong sodium bicarbonate secretions against acidic water, acidic food, acidic air, and acidic thoughts.
These glands and organs are the salivary glands, the stomach, the pancreas, the Brunner’s glands (between the pylorus and the junctions of the bile and pancreatic ducts), the Lieberkuhn’s glands in the liver and its bile with its strong acid binding capabilities which it has to produce.
5) When a rich animal protein and/or carbohydrate meal (like meat and potatoes, spaghetti bolognaise etc.) is ingested the stomach begins to manufacture and secret sodium bicarbonate or NHCO3 to alkalize the acids from the food ingested.
This causes a loss in the alkaline reserves in the blood and interstitial fluids and an increase in acid and/or HCL found in the gastric pits of the stomach.
These acids and/or HCL are eventually taken up by the blood which lowers blood plasma pH. The blood eliminates this increase in gastro-intestinal acid by throwing off into the Pishinger’s spaces or compartments of the Interstitium.
6) The space enclosed by these finer and finer fibers is called the Pishinger’s space or the extra-cellular space or the compartments of the Interstitium that contains the fluids that bath and feed each and every cell while carrying away the waste from those same cells.
There is no mention of this organ in American medical physiology text books. There is the extra-cellular fluids or interstitial fluids but no organ mentioned that stores acids from metabolism and diet, like the kidney. I call this organ the “pre-kidney” or the Interstitium, which is the largest organ of the body only just now being recognized by American medical science (2018).
7) After a rich animal protein or sugary meal, the urine pH becomes alkaline.
Protein and sugar nourishment then reacts acidic in the organism not only by the production of sulfuric, phosphoric, nitric, uric, lactic and acetyl aldehyde acids, respectively, but also through the formation and excretion of base or alkalinity in the urine. The ingestion of animal protein causes a double loss of base or alkalinity and why it is so dangerous to ingest.
8) During heavy exercise, the resulting lactic acid if not adsorbed by the collagen fibers or connective tissues, the specific acid catchers of the body, the organism would die. The total collection of these fibers is the largest organ of the body and is called the colloidal connective tissue organ of SCHADE also known as of 2018 the Interstitium.
NO liquid exchange occurs between the blood and the parenchyma cells, or in reverse, unless it passes through this connective tissue organ called the Interstitium, the largest organ in the human body.
This organ connects, holds everything in our bodies in place. It is composed of ligaments, tendons, sinew, and the finer fibers that become the scaffolding that holds every single cell in our bodies in place.
When acids are stored in this organ, which includes the muscles, inflammation and pain develop.
That is why I have stated for over 30 years, “acid is pain and pain is acid.”
You cannot have one without the other.
This is the beginning of blood plasma, interstitial fluid and intracellular acidosis.
9) The more acidity in the food we eat, the water we drink, the air we breath and the thoughts we conceive the more acids that are adsorbed to the collagen fibers to be neutralized or buffered, and the less sodium bicarbonate or NaHCO3 is taken up by the alkalizing or alkalophile glands.
The larger the potential difference between the adsorbed acids into the blood and then Interstitium and the amount of NaHCO3 generated with each meal or thought; the more or less alkaline or rich in bases are the alkalophile glands like the pancreas, gallbladder, pylorus glands, blood, etc. The acid binding power of the connective tissue, the blood, the Intestitium and the alkalophile glands depends on its alkali reserves which can be determined through blood, urine, saliva and interstitial pH testing, including live and dried blood analysis.
10) The iso-structure of the blood attempts to maintain its delicate pH at 7.365 by pushing off the acidic waste from lifestyle and diet into the connective tissue of the Schade or now referred to as the Interstitium.
The blood gives to the urine the same amount of acid that it receives from the Interstitium, tissues and liver so it retains its iso-form and pH of 7.365.
A base or alkaline deficiency is always related to the deterioration of the deposit ability of the connective tissues and the Interstitium.
As long as the iso-structure of the blood pH is maintained, the urine, which originates from the blood, remains a faithful reflected image of the acid-base regulation, not of the blood, but of the interstitial fluids of the Interstitium and the cells that make up the organs, tissues and glands.
The urine therefore is an excretion product of the tissues and Interstitium and not the blood.
So when you are testing the pH of the urine you are actually testing the pH of the intracellular fluids of the organs, glands and tissues as well as the interstitial fluids of the Interstitium.
11) A latent (delayed) “acidosis” is the condition that exists when there are not enough bases in the alkalophile glands because they have been used up in the process of neutralizing the acids adsorbed to the collagen fibers and the Interstitium.
This leads to compensated “acidosis” in the Interstitium and not in the blood.
This means the blood pH has not changed but other body systems have changed. This can then lead to de-compensated “acidosis” where the alkaline reserves of the blood are used up and the pH of the blood is altered and caused by what you are eating, drinking, breathing and thinking.
De-compensated “acidosis” can be determined by testing the blood pH, interstitial fluid pH, urine pH and the saliva pH.
The decrease in the alkaline reserves in the body occurs because of hyper-proteinization, (eating the flesh of animals!) too much protein and hyper-carbonization, too much sugar.
This is why 80 to 90 year old folks are all shrunk up and look like prunes.
They have very little or no alkaline reserves in their alkalophile glands and the interstitial fluids of the Interstitium have become highly acidic!
When all the alkaline minerals are gone (sodium, potassium, magnesium and calcium) so are you and your electron battery runs down. The charge of your cellular electron battery can be measured testing the face angel of the cells, the ORP or the oxidative reduction potential of the blood and interstitial fluids. The test is called the 3D Bio-Electro scan.
As you become more acidic in the Interstitium this energy potential or ORP decreases. Remember your body cells rung on electrons and NOT sugar, protein or fat! We are electrical beings by function releasing chemical waste physiologically – like lactic or citric acid.
12) If there is not enough base left over after a protein or sugary meal, or enough base to neutralize and clear the acids stored in the connective tissues and the Interstitium, a relative base deficiency develops which leads to systemic tissue and interstitial fluid acidosis leading to decompensated acidosis of the blood.
When this happens the liver and pancreas are deficient of adequate alkaline juices of sodium bicarbonate to ensure proper alkalization of the food in your stomach and small intestine. This leads to genetic and stem cell mutations, deficiencies in red blood cell production in the crypts of the small intestines and finally deficiencies in the body cells that make up all glands, tissues and organs or your body.
13) Digestion or alkalization cannot proceed without enough of these alkaline juices for the liver and pancreas, etc., so the stomach has to produce more acid in order to make enough base or sodium bicarbonate leading to ad nauseam. If this biological deficiency continues then one can develop indigestion, nausea, acid reflux, GERD, ulcers, esophageal cancer, stomach cancer and bowel congestion, food allergies, constipation, diverticulitis, diverticulosis, inflammation of the bowels and finally colon cancer.
All of these symptoms are not the result of too much acid, on the contrary, it is the result of too little base or alkalinity and the major reason for drinking high pH alkaline water at 9.5!

14) The stomach is NOT an organ of digestion as currently taught in ALL biology and medical texts, BUT an organ of contribution or deposit of the alkaline compound, sodium bicarbonate.
Its function is to deposit alkaline juices to the stomach to alkalize the food, water and to the blood, to carry sodium bicarbonate to the alkalophile glands!!!! The stomach is also assigned to maintain the delicate pH of the blood and interstitium at 7.365 and the main reason I suggest drinking high pH alkaline water with your meals to buffer the highly acidic and toxic HCL.

15) There is a daily rhythm to this acid base, ebb and flow of the fluids of the body. The stored acids are mobilized from the connective tissues and Pishinger’s spaces of the Intestitium while we sleep.
These acids reach their maximum (base tide) concentration in the blood and interstitial fluids, and thereby the urine at 2 am is the most acidic.
The acid content of the urine directly reflects the acid content of the fluid in the Pishinger’s spaces of the Interstitium, the extra-cellular fluid compartments of the body protecting the delicate pH balance of the blood at 7.365.
On the other hand the Pishinger’s spaces of the Interstitium becomes the most alkaline around 2 pm (the base flood) as then the most sodium bicarbonate or NaHCO3 is being generated by the cover cells of the stomach to alkalize what we eat, drink, breath and think.
16) If your urine is not alkaline by 2 pm you are definitely in an ACIDIC condition and lacking in the necessary alkaline reserves, especially sodium, chloride, potassium, magnesium and calcium.
The pH of the urine should ideally run between 7.2 and 8.4 when maintaining a healthy state of the blood, interstitial fluids of the Interstitium and the Intracelluar fluids at a pH of 7.365.
17) The free flowing acids formed after a high animal protein meal include sulfuric, phosphoric, uric and nitric acids that will stick to the collagen fibers of the Interstitium to remove them from the blood and protect the blood’s delicate pH of 7.365.
The H+ or proton ions from these acids are neutralized by the next base flood, the sodium bicarbonate produced after the ingested acidic meal.
The H+ or proton ion combines with the carbonate or HCO-3, converts to carbonic acid, H2CO3, which converts to CO2 and H2O.
The sulfuric and other toxic acids from proteins are neutralized as follows where the HR represents any acid with the R as its acidic radical (SO4, PO4, or NO3) HR + NaHCO3 <=> H2O + NaR (Ca, Mg, K)+ CO2.
18) Medical doctors, Naturopathic doctors and savants do not recognize interstitial fluid and tissue acidosis because they never studied it in medical or naturopathic college. This is why they make statements that what you eat or drink does not matter because you cannot change the pH of the blood. This only shows their ignorance and lack of understanding of the anatomy and physiology of the stomach, pancreas, gallbladder, blood and Interstitium.
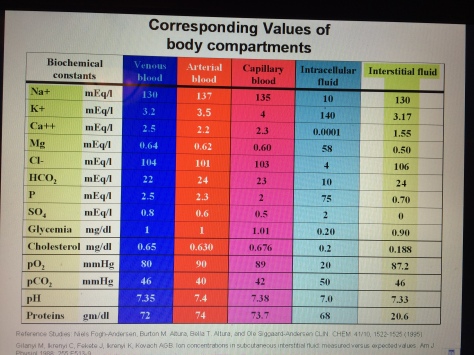
Currently medical and naturopathic doctors do recognize compensated acidosis and de-compensated acidosis but they DO NOT understand or recognize latent tissue acidosis which takes place in the Interstitium. Why? Because they no very little about this organ system and they do not have the technology or the equipment to test its chemistry, including pH and compare this quantitative information with the chemistry of the blood. This is not only dangerous it is also non-intelligent because the Doctor is basing your condition on the results of only 10 to 20 percent of your body fluids – the blood. Hospitals and clients around the World DO NOT test 80 to 90 percent of the body fluids which include the interstitial fluids of the Interstitium and the Intracellular fluids. The chemistry, including the pH of the Interstitium is important to the over-all understanding of what is really going on when the body has health challenges.
In compensated acidosis breathing increases in order to blow off more carbonic acid which decreases PCO2 because of the lowered carbonate or HCO3.
When the breathing rate can no longer get any faster and when the kidneys can no longer increase their function to keep up with the acid load then the blood pH starts to change from a pH of 7.365 to 7.3 then to 7.2. At a blood pH of 6.95 the heart relaxes and the patient goes into a coma and dies.
19) Metabolism of a normal adult diet results in the generation of 50 to 100 meq of H+ or proton per day, which must be excreted if the urine acid-base balance is to be maintained at 7.2 or greater and the blood and interstitial fluids of the Interstitium are to be maintained at 7.365. Currently myself and Dr. Galina Migalko are the only published scientists in the World on this subject of upmost importance in medical diagnostics and correct and effective treatments that can be quantified and validated in their efficacy!
A meq is a milli-equivalent which is an expression of concentration of substance per liter of solution, calculated by dividing the concentration in milligrams per 100 milliliters by the molecular weight.
This process involves two basis steps;
1) the re-absorption of the filtered sodium bicarbonate or NaHCO3 and,
2) the excretion of the 50 to 100 meq of H+ or proton or acids produced each day by the formation of titratable acidity and NH4+ or ammonium.
Both steps involve H+ or proton or acid secretion from the cells of the kidney into the urine which can then be measured using pH Hydrion strips.
The loss of NaHCO3 in the urine is equivalent to the addition of H+ or acidity to the blood, Interstitium and body cells, since both are derived from the dissociation of H2CO3 or carbonic acid.
21) The biochemistry is: CO2 + H2O = H2CO3 = HCO3 + H+.
The normal person must reabsorb 4300 meq of NaHCO3 each day which eventually will lead to aging and death.
The secreted H+ or proton ions are generated within the kidney cells from the dissociation of H2O or water.
This process also results in the equimolar production OH- or hydroxyl ions or alkalinity.
The OH- ions bind to the active zinc-containing site of the intracellular carbonic anhydrase; they then combine with CO2 to form HCO3-ions, which are released back into the kidney cells and returned to the systemic blood circulation.
Second, the dietary acid load is excreted by the secretion of Hydrogen or H+ or proton ions from the kidney cells into the urine.
These H+ or proton ions or acids can do one of two things:
The H+ or proton ions or acids can combined with the urinary buffers, particularly HPO4 in a process called titratable acidity.
The biochemistry is:
H+ + HPO4 = H2PO4, or the phosphate buffering system or the H+ or proton ions can combine with ammonia (NH3) to form ammonium as follows:
NH3 + H+ = NH4.
22) This ammonia is trapped and concentrated in the kidney as ammonium, which is then excreted in the urine.
23) In response to the acid load in the body fluids:
36% of the Hydrogen or H+ or proton or acid goes intracellular in exchange for the release of Na+ (sodium) into the blood stream.
15% of the acid load goes intracellular in exchange for K+ (potassium) – common in diabetics.
6% of the Hydrogen or H+ or proton or acid goes directly into the cell to be buffered by intracellular processes. This is what causes cell mutations that leads to degenerative disease.
43% is buffered extra-cellularly or in the Interstitium as NaHCO3 or sodium bicarbonate combining with Hydrogen or H+ or proton to form H2CO3 or carbonic acid which breaks down to CO2 or carbon dioxide to be released by the lungs.
10% of CO2 or carbon dioxide is excreted through the lungs and 90% of CO2 is used by the body to re-absorb alkaline minerals and make sodium bicarbonate. The biochemistry is: CO2 + H2O = H2CO3 = HCO3 + H+.
24) Of all the ways the body can buffer metabolic and dietary acids to protect the blood, interstitial and intracellular pH, is the excretion of protein (the eating of animal flesh) generated acid residues is the only process that does not add sodium bicarbonate back into the blood circulation.
This creates a loss of bases or alkalinity in the blood, Interstitium and Intracellular fluids which is the forerunner of ALL sickness and disease.
In the long run the only way to replace these lost bases is by eating more alkaline electron-rich green food, drinking alkaline water at a pH at 9.5 and -250 mV, supplementing sodium, potassium and magnesium salts and long chain polyunsaturated fats on a daily basis.
Remember, a cucumber a day keeps the Doctor away – an apple a day creates more acidic waste leading to blood, interstitial fluid and tissue acidosis.
25) Very important to remember: The Human Body is an acid-producing organism by function, yet it is an alkaline organism by design when healthy and strong.
Eating animal protein and sugar are deadly acidic choices – unless you want to risk premature sickness, disease and the an early painful death.
References:
1) Sick and Tired by Robert O Young DSc, PhD, Naturopathic Practitioner
2) The pH Miracle revised and updated by Robert O Young DSc, PhD, ND
3) Alkalizing Nutritional Therapy in the Prevention and Reversal of Any Cancerous Condition by Robert O Young DSc, PhD, Naturopathic Practitioner and Galina Migalko MD, NMD

4) A NEW THEORY – THE PHYSIOLOGY OF THE STOMACH: An Alkalizing Organ by Design and Function and NOT an Acidic ORGAN of DIGESTION by Robert O Young, DSc, PhD, Naturopathic Practitioner











Thank you for your information. I have cancer and am looking at your info which I find very interesting.
LikeLike